Acute myeloid leukemia (AML) is a type of acute leukemia that affects myeloid cells. It is characterized by uncontrolled proliferation of immature WBCs causing an accumulation of immature WBCs in the bone marrow and peripheral circulation. AML is an aggressive cancer that can be life-threatening if not treated promptly, therefore, early detection is crucial. Identification of immature WBCs and their subtypes is the first step towards AML diagnosis. Microscopic blood smear image analysis is commonly used to diagnose AML as it is less expensive non-invasive diagnostic tool compared to bone marrow biopsy and immunophenotype. Nonetheless, classifying immature WBCs presents challenges due to their high similarity and minimal interclass variation, especially for intermediate stages of myelopoiesis which make is susceptible to misclassification. The current diagnostic methods of AML are based on manual identification of immature WBCs using peripheral blood smear. These methos are time-consuming, prone to errors, and subject to inter-observer variation. Therefore, this study aims to develop a computer-aided diagnostic framework to accurately identify immature WBCs and classify them into various subtypes.
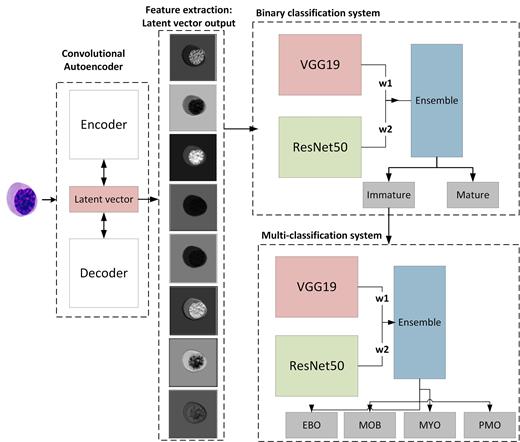
The proposed framework consists of two main components: a binary classification system to classify cells into mature versus immature cells and a multiclassification system to further classify immature cells into four subtypes including myeloblast, monoblast, and promyelocytes. The proposed framework was designed to undergo four distinct phases: the first phase involves preprocessing, which includes data augmentation techniques aimed at addressing the imbalance distribution of WBCs. Augmentation encompasses geometric transformation and generation of synthetic images using convolutional Autoencoder (CAE). Feature extraction phase involves using image embedding and transfer-learning. Image embedding representation was obtained using CAE and then utilized by two pre-trained models: VGG19 and Resnet50. Classification phase was carried out using weighted ensemble of the VGG19 and Resnet50 with the optimal weights determined through Grid search. The validation phase evaluated the model performance using overall accuracy, precision, and sensitivity. The area under the ROC curve (AUC) was used to assess model discrimination ability.
The proposed framework demonstrated excellent results, achieving an average accuracy of 99.5%, a sensitivity of 97.97%, and a precision of 94.12%. The model exhibited an excellent discriminatory capability, with an AUC of 99.9%. Furthermore, the proposed framework outperformed previous methods, showcasing improved performance.
Key word:
Acute myeloid leukemia, peripheral blood smear, immature white blood cells, deep learning, feature extraction, computer-aided diagnosis.
Disclosures
No relevant conflicts of interest to declare.